



Reagents:
A series of tubes needs to be prepared (standard solutions) with known decreasing concentrations of acetoacetate: 16, 8, 4, 1.5 and 0.5 mM.
Each standard solution will be preparaed by dilution of the previous one. The total volume of each one must be between 200 and 500 µL.
| tube number |
desired concentration |
previous standard |
volume to take from the previous standard solution (µL) |
volume of water (µL) to add |
|
|---|---|---|---|---|---|
| P1 | 16.0 mM | P0 | |||
| P2 | 8.0 mM | P1 | |||
| P3 | 4.0 mM | P2 | |||
| P4 | 1.5 mM | P3 | |||
| P5 | 0.5 mM | P4 |
You may then if your procedure is correct. All volumes are correct. 👍




In this experiment we intend to semiquantitatively estimate the concentration of ketone bodies in urine samples m1, m2 and m3.
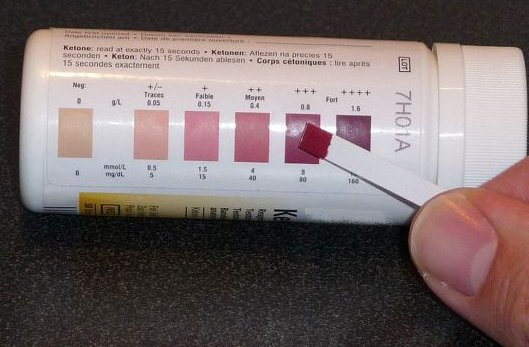
In you lab notebook: write down the code asigned to your particular experiment (in this case it is and which of the samples (m1, m2 or m3) you have applied onto each reactive strip (A, B, C). By comparing the colour displayed with those of the standards, estimate the concentration of ketone bodies in each sample. Finally, interpret your results: Is there ketosis? Signs of diabetes?
Acetoacetate is detected through a reaction with nitroprusiate that forms a coloured product:
 sodium nitroprusside
sodium nitroprusside

![]() Offered for use under the terms of the Creative Commons Attribution – NonCommercial – ShareAlike License.
Offered for use under the terms of the Creative Commons Attribution – NonCommercial – ShareAlike License.
Programmed in HTML5, CSS and JavaScript and foreseeably compatible with all types of web browsers, operating systems and devices. Features are used from the open surce libraries Dragula (by bevacqua, MIT License) and ColorMix (by Flo-Schield-Bobby, MIT License).