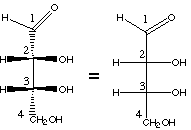
 |
eclipsed conformation |
|
| Fischer projection | D-erythrose | Newman projection |
 |

eclipsed conformation |
|
| Fischer projection | D-erythrose | Newman projection |
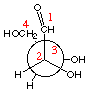
Exercise 2.6If you have the means available (*), prepare a physical model of D-threose to help you.Draw a Newman projection for D-threose in the eclipsed conformation, similar to the one displayed above for D-erythrose. |
D-threose |