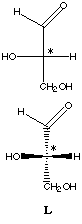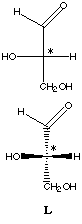Introduction
Enantiomeric forms of glyceraldehyde are used as the reference to define the D-L nomenclature system for stereoisomers.
Glyceraldehyde has a single chiral carbon (labeled in these models with an asterisk). Its enantiomers differ only on the spatial orientation of the hydroxyl (-OH) and hydrogen (-H) groups bound to C*. Move the models around to convince yourself that they are not identical.
|
D-glyceraldehyde |
|
L-glyceraldehyde |
|
 |
|
|
|
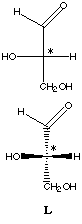 |
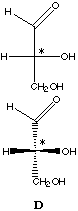
The Fischer projection formula is a condensed form of drawing the three-dimensional formula using wedges.
In this formula, groups located on the horizontal axis (in this case, -H and -OH
bound to C*) protrude towards you.
Exercise 2.1
Indicate which are the R-S configurations for D and L glyceraldehyde.